Researchers Uncover Mechanisms That Impair Immune System Defenses
Core processes suppressing anti-viral immunity could be relevant in cancer attacks
April 26, 2018
By Mario C. Aguilera
The human immune system maintains intricate defenses against a constant wave of attacks from pathogens, but such protections are not limitless. Eventually, these defenses lose their effectiveness or simply stop working against attacks from viruses, bacteria, parasites and even tumors.
University of California San Diego Biological Sciences Professor Elina Zúñiga and members of her lab have been investigating how cells in the immune system adapt to these microbial invaders.
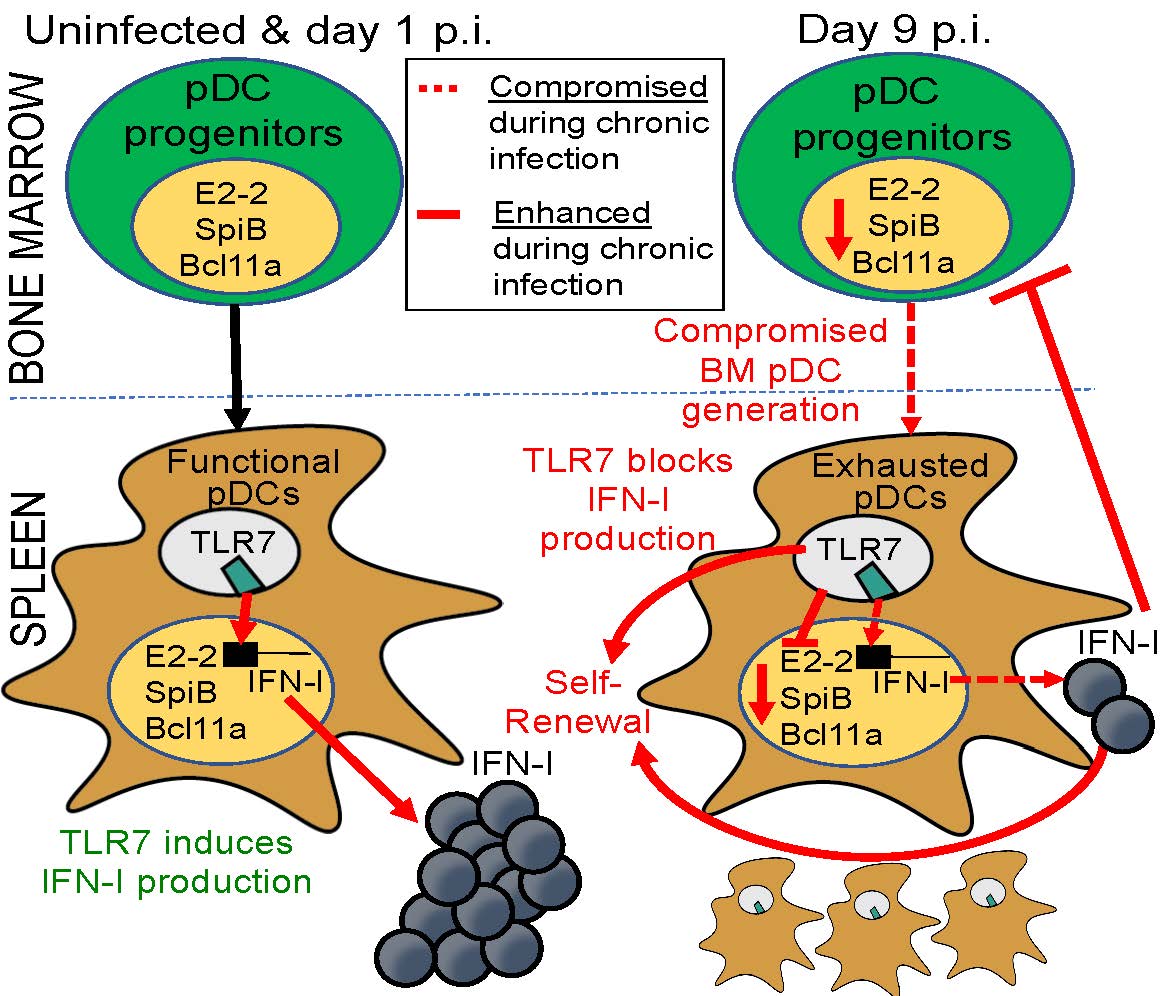
In a recent issue of the journal Immunity, Zúñiga, co-first authors Monica Macal and Yeara Jo and their colleagues describe the mechanisms underlying adaptations of one class of short-lived innate cells against chronic inflammation. So-called plasmacytoid dendritic cells (pDCs) play crucial roles in the first line of defense by producing copious amounts of type I interferons (IFN-I), proteins that provide resistance to infections and tumors. Previous work from Zúñiga’s team and others has shown that during chronic viral infection and cancer episodes, pDCs become functionally exhausted and lose their IFN-I production capacity, but the underlying mechanisms remained elusive. In the new study, the researchers pinpoint multiple processes in bone marrow and spleen that maintain a pool of functionally exhausted pDCs that ultimately compromise immune defense.
Strikingly, the keys to pDC exhaustion or suppression, the researchers found, were IFN-I and a toll-like receptor known as TLR7, which in other contexts promote pDC function. During chronic infection, however, these two players exerted previously unappreciated effects maintaining a self-perpetuating pool of functionally exhausted pDCs. The researchers say the new findings offer a framework to decipher long-term exhaustion of other types of short-lived innate cells during episodes of chronic inflammation, with possible applications for fighting infections and cancer.
“If you understand the mechanisms by which pDCs become exhausted and lose their function, as well as how they might be maintained, it may be possible to intervene to empower these cells to rebound interferon production and continue with vital immune defenses,” said Zúñiga. “In this study we show how—if we prevent functional exhaustion of these cells by genetically deleting TLR-7—we can better equip defenses against secondary infections, which can be life-threatening complications in patients suffering from chronic infections.”
Zúñiga says the fundamental biology behind this mechanism might be extended to the tumor environment that also suppresses immune defenses.
“If we understand how cells respond to chronic infection, then we can hopefully manipulate the pathways leading to suppression to empower the immune cells to fight infected cells or potentially fight cancer cells,” said Zúñiga.
Coauthors of the paper include Simone Dallari, Aaron Chang and Ellen Wehrens of UC San Diego Biological Sciences; and Jihong Dai, Shobha Swaminathan and Patricia Fitzgerald-Bocarsly of Rutgers New Jersey Medical School.
The research was supported by a scholar grant from the Lupus Research Institute, a scholar research grant from the American Cancer Society and National Institutes of Health grants AI081923, AI113923 and AI106125.
